Blood Pressure Meds Recalled
The pharmaceutical industry is no stranger to recalls due to a plethora of serious health problems including cancer. Updated 1355 9 Aug 2021.

Blood Pressure Heart Meds Recalled Due To Health Risks Amandala Newspaper
A blood pressure medication is being recalled for possibly containing high levels of a cancer-causing impurity according to a recall notice from the US.

Blood pressure meds recalled. MONTREAL -- A trio of high blood pressure medications are being recalled due to the presence of an impurity. Continue Reading Show full articles without Continue Reading. Dozens of blood pressure medications have been recalled since the first products were pulled off the shelf in July 2018 due to impurities.
FDA has issued a recall of certain lots of angiotensin II receptor blocker ARB high blood pressure medication containing valsartan losartan or irbesartan. 0914 18 Jun 2021. Health Canada has recalled some lots of blood pressure drugs under the names irbesartan losartan and valsartan as some may contain what.
Overall Torrent has recalled more than. A common blood pressure drug is being recalled over fears of cancer-causing chemicals. Food and Drug Administration FDA.
Blood pressure and fluid retention drugs recalled over cancer concerns. The American Heart Association has more on high blood pressure medications. The medications are generally used to treat high blood pressure and heart failure but.
This includes some combination tablets which contain valsartan and amlodipine or valsartan amlodipine and hydrochlorothiazide. Food and Drug Administration FDA for potentially containing a probable human carcinogen. Lupin Pharmaceuticals is voluntarily recalling two types of blood pressure medications after certain batches were shown to.
In fact aside from several medications medical devices have also been recalled due to their. Click here to read the full article. The post FDA blood pressure medicine recall.
Makers of blood-pressure medication expand their recall. 16 hours agoA blood pressure medication is being recalled for possibly containing high levels of a cancer-causing impurity according to a recall notice from the US. COMMON blood pressure drugs have been recalled over contamination fears with a substance that can increase the risk of cancer.
The FDA has published a new recall advisory from Lupin Pharmaceuticals over two different drugs. Common blood pressure drugs are being recalled by the UK medicine regulator over fears of contamination with a substance that can increase the risk of cancer over time. The UK medicine regulator.
The affected products all contained valsartan losartan. The Medicines and Healthcare products Regulatory Agency MHRA issued a. Blood pressure meds recalled.
Home News Blood Pressure Meds Recalled Over Cancer Concerns Blood Pressure Meds Recalled Over Cancer Concerns October 19 2021 Erica Davies News 0 comments. The blood pressure medication was recalled after it was discovered that the bottles may contain 40-mg pills instead of 20-mg pills thus potentially. A Lupin Pharmaceuticals Inc.
The expiration date of the medication is March 2022. 1170 rows Search List of Recalled Angiotensin II Receptor Blockers ARBs including Valsartan. Torrent said its only recalling lots containing NMBA above what the FDA considers acceptable for daily use.
12 hours agoA Lupin Pharmaceuticals Inc. The recalled Telmisartan tablets have a lot number of 1905005661 and an NDC number of 62332-087-30. The blood pressure medication was recalled after it was discovered that the bottles may contain 40-mg pills instead of 20-mg pills thus potentially jeopardizing the health and safety of those who take them.
Instead patients should talk to their pharmacist or physician about an alternative treatment and should contact their healthcare provider if theyve experienced any problems that may be related to the recalled valsartan products the company advised. The recall of Losartan is linked to a possible cancer-causing element known as NMBA. The Food and Drug.
Dozens of medications used to treat high blood pressure including valsartan losatran and irbersartan have been recalled over the past several months as federal investigators discover. Blood pressure drug Losartan recalled 0029. Blood pressure medication is being recalled by the US.
Stop taking these pills now appeared first on BGR. According to the FDA notice Patients who could be on a doubled dose of telmisartan for a prolonged period of time could experience low blood pressure worsening of kidney function or an. By Connie Lin 1 minute Read.

Blood Pressure Meds Recalled Over Contamination Concern Wfmj Com

Blood Pressure Drugs Recalled By 2 Companies Masslive Com
/cloudfront-us-east-1.images.arcpublishing.com/gray/HJZ5B42YZNEJNDWE6MAMLCX2FA.jpg)
Blood Pressure Medication Recalled Due To Unexpected Impurity

Blood Pressure Drug Recalled After Probable Cancer Causing Ingredient Detected Fox News

Blood Pressure Drug Recalled Over Risky Label Mix Up Everyday Health
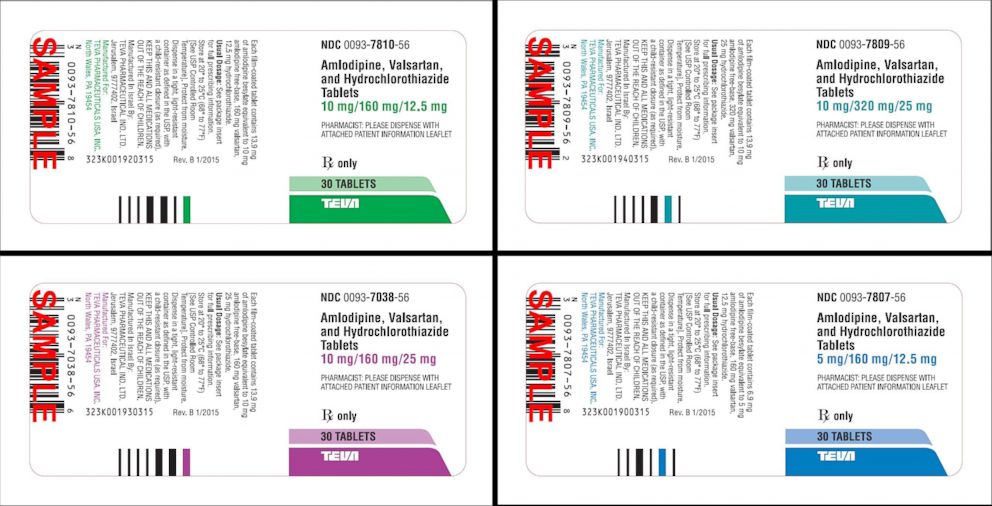
Blood Pressure Medication Recall What You Need To Know Abc News

Cancer Causing Chemicals Spark Recall Of Blood Pressure Drugs Over Contamination Fears

Blood Pressure Medication Recalled Because May Contain Stronger Dose Than Indicated Pennlive Com
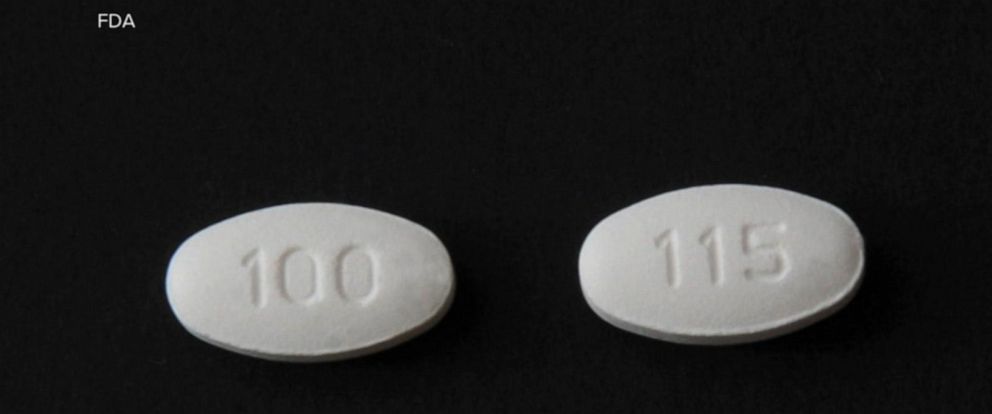
Blood Pressure Medication Recall What You Need To Know Abc News

Blood Pressure Medication Recalled For Label Mix Up Silive Com

Common Blood Pressure Drug Recalled Due To Cancer Causing Chemicals Wales Online

Recall Expanded For Blood Pressure Medication Due To Potential Cancer Risk Abc7 Los Angeles
Blood Pressure Medication Recall What You Need To Know Abc News
/cloudfront-us-east-1.images.arcpublishing.com/gray/A7A5CIT5GREO7OVZPSG4ZHG2U4.png)

/cloudfront-us-east-1.images.arcpublishing.com/gray/IFRWTBMGXFAAVL36BTAQD77TQ4.png)

