Blood Pressure Pills Recalled
As pharmaceutical companies have worked on. Health chiefs recall another 25 batches of blood pressure pills because they contain an impurity that may cause cancer.

High Blood Pressure Drug Recall Expands Again Over Cancer Risk Slashgear
You may have questions we have answers.

Blood pressure pills recalled. April 07 2020 - NDEA designated as a Human Carcinogen - One of the impurities which have been found in the recalled high blood pressure medications is NDEA or Nitrosodiethylamine. COMMON blood pressure drugs have been recalled over contamination fears with a substance that can increase the risk of cancer. According to the FDA those recalls which began in June of 2018 involve a number of angiotensin II receptor blockers ARBs a type of medication used to treat high blood pressure which include.
Dhiraj SinghBloomberg via Getty Images. If you take a medication that has been recalled your healthcare provider will discuss alternative medications to. This includes some combination tablets which contain valsartan and amlodipine or valsartan amlodipine and hydrochlorothiazide.
Blood pressure medication is being recalled by the US. Over the past three years millions of blood pressure pills that contain a probable carcinogen have been recalled around the world. The recalled medications include the generic versions of amlodipine valsartan losartan irbesartan and hydrochlorothiazide.
One to watch. The recalled Telmisartan tablets have a lot number of 1905005661 and an NDC number of 62332-087-30. The UK medicine regulator today issued a recall for 31 batches of prod.
Blood pressure pill recall. Were voluntarily recalled by the manufacturer after routine ingredient testing detected high levels of N. DOZENS of blood pressure pills have been recalled from pharmacy shelves because they contain a chemical linked to cancer.
Unfortunately patients taking the popular medication were left vulnerable to the very conditions the drugs claimed to treat and exposed to additional threats. Cancer-causing chemicals spark recall of blood pressure drugs over contamination fears but patients have been warned not stop taking their medications. The Medicines and Healthcare products.
The expiration date of the medication is March 2022. The blood pressure medication was recalled after it was discovered that the bottles may contain 40-mg pills instead of 20-mg pills thus potentially jeopardizing the health and safety of those who take them. Batches of blood pressure pills were recalled today after a cancer-causing chemical was found in them.
Blood pressure pills recalled. Fears that some drugs have been contaminated with a substance that could possibly increase the risk of cancer has seen certain blood pressure drugs recalled. A trio of high blood pressure medications are being recalled due to the presence of an impurity.
Blood Pressure Pills Recalled Over Cancer Risk Medications may contain high levels of a probable human carcinogen. You may have recently heard that FDA has recalled several blood pressure medications. Dont stop your medicine without contacting your healthcare provider The risk of abrupt discontinuation of BP drugs can be significant.
The recall has been issued by the Medicines and Healthcare products. NDEA is an organic chemical which can be toxic to the liver as well as other. If you or a family member took Losartan or a related drug and developed.
Dozens of blood pressure medications have been recalled since the first products were pulled off the shelf in July 2018 due to impurities. It is a precautionary measure to. The UK medicine regulator has issued a recall for 31 batches of Irbesartan-containing products and two batches of Losartan products.
After the discovery pharmacies stocking the implicated medications - several different kinds of irbesartan and losartan - were ordered to withdraw them. Torrent Pharmaceuticals is expanding a recall of blood-pressure medication possibly tainted with a cancer-causing chemical. The affected products all contained valsartan losartan.
Two types of blood pressure medications sold by Lupin Pharmaceuticals Inc. Many people counted on these drugs to keep them healthy after suffering a stroke or heart attack or lower their high blood pressure to prevent a major heart event. A BATCH of common blood pressure pills has been recalled following concerns the drug may contain cancer-causing chemicals.
What to do if you have these medicines in your cabinet or store News Instacart is acquiring a maker of smart grocery carts wading deeper into physical retail. Health Canada has recalled some lots of blood pressure drugs under the names irbesartan losartan and valsartan as some may contain what Health. Common blood pressure drugs are being recalled by the UK medicine regulator over fears of contamination with a substance that can increase the risk of cancer over time.
By Solen Le Net 1612 Mon Aug 9 2021. 0914 18 Jun 2021. According to the FDA notice Patients who could be on a doubled dose of telmisartan for a prolonged period of time could experience low blood pressure worsening of kidney function or an.
Continue Reading Show full articles without. By Aaron Kassraie AARP October 18 2021. Common drugs used to treat high blood pressure have been recalled by the UK s medicines agency after being contaminated.
14 hours agoBlood pressure medication recalled over possibly containing cancer-causing impurity 0 shares A Lupin Pharmaceuticals Inc.
Nationwide Recall Of Certain High Blood Pressure And Heart Failure Medications Issued Wztv
Fda Recalls Blood Pressure Medicine After Finding Carcinogen In Pills Phillyvoice

New Generic Blood Pressure Drug Is Approved To Relieve Shortages The New York Times
Blood Pressure Medication Recall What You Need To Know Abc News

Cancer Causing Chemicals Spark Recall Of Blood Pressure Drugs Over Contamination Fears

Blood Pressure Drug Recalled Over Risky Label Mix Up Everyday Health
Valsartan Losartan And Other Blood Pressure Medication Recalls 2018 19

Blood Pressure Medication Recalled For Cancer Risk

More Blood Pressure Pills Recalled Over Cancer Causing Chemical

Blood Pressure Pills Recalled Because They Contain World S Most Explosive Chemical Which Also Causes Cancer The Bl
Alert Recall Of Other High Blood Pressure Medicines Crossville News First
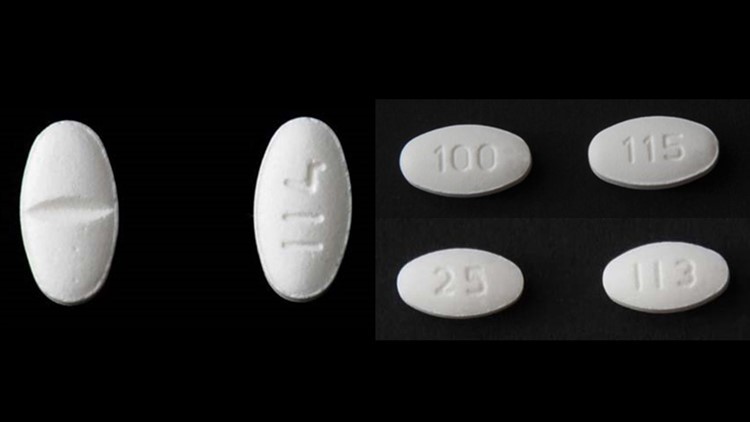
More Blood Pressure Medications Recalled For Cancer Causing Impurity Wtol Com

Blood Pressure Pill Recalled After Possible Life Threatening Labeling Error
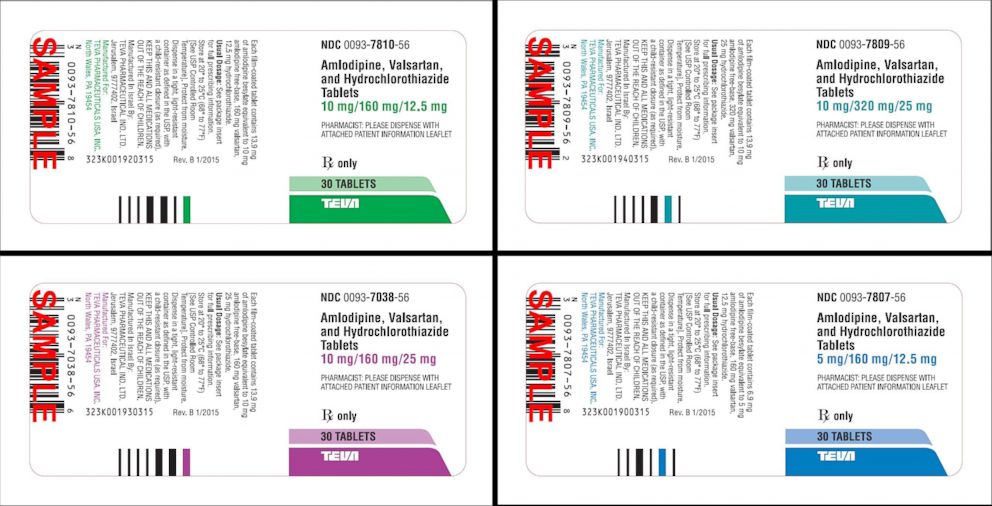
Blood Pressure Medication Recall What You Need To Know Abc News

Blood Pressure Medicine Recall Expanded For Fifth Time Wkbn Com
Blood Pressure Medication Recall Expands Again To Include Losartan Wlos