Blood Pressure Meds Recalled For Causing Cancer
All of the affected medicines contain irbesartan which is used. Torrent Pharmaceuticals is expanding a recall of blood-pressure medication possibly tainted with a cancer-causing chemical.
Roston - Oct 15 2021 744pm CDT.

Blood pressure meds recalled for causing cancer. 18 2021 -- Two types of blood pressure medication made by Lupin Pharmaceuticals have been recalled due to potential high levels of a cancer-causing substance according to an FDA recall. Is voluntarily recalling its Irbesartan and Hydrochlorothiazide tablets at the consumer level. More Blood Pressure Medication Recalls Due To Cancer Concerns.
October 19 2021 439 pm EST Filed under. Are being recalled due to concern with cancer causing impurities in the. Another 25 batches of high blood pressure pills have been recalled over fears they may cause cancer health chiefs announced today.
Blood pressure medication recalled over possibly containing cancer-causing impurity FDA made the assessment of Lupin Pharmaceuticals product after laboratory testing. Blood Pressure Meds Recalled Over Cancer Concerns. Updated 1355 9 Aug 2021.
A blood pressure medication is being recalled for possibly containing high levels of a cancer-causing impurity according to a recall notice from the US. Health Fitness by News Staff. The Food and Drug Administration has recently recalled a number of blood pressure medications after discovering that they contained potential cancer-causing contaminants.
A Food and Drug Administration recall of a heart medication due to a cancer-causing chemical now includes two blood pressure medications. Why Colin Powells death after COVID-19 vaccination is rare Colorado Springs Colorado. Blood pressure drugs recalled over fears they contain cancer-causing chemical.
A carcinogen is something that could cause you to have cancer. The FDA has published a new recall advisory from Lupin Pharmaceuticals over. Patients are being advised not to stop taking their medication as it.
COMMON blood pressure drugs have been recalled over contamination fears with a substance that can increase the risk of cancer. Is voluntarily recalling its Irbesartan and Hydrochlorothiazide tablets at the consumer level. Blood pressure medication recalled over risk of cancer-causing impurity Cleveland Ohio.
Cancer-causing chemicals spark recall of blood pressure drugs over contamination fears but patients have been warned not stop taking their medications. Blood pressure and fluid retention drugs recalled over cancer concerns. A common blood pressure drug is being recalled over fears of cancer-causing chemicals.
Teva Pharmaceuticals has issued a voluntary recall of its. The recall has been issued by the Medicines and Healthcare products Regulatory Agency MHRA and concerns 25 batches of Irbesartan-containing medications. Fears that some drugs have been contaminated with a substance that could possibly increase the risk of cancer has seen certain blood pressure drugs recalled.
January 23 2019 1255 PM CBS News. Blood Pressure Meds Recalled Due to Cancer Causing Impurity. Food and Drug Administration FDA.
Food and Drug Administration FDA. More than 30 batches of pills used to treat high blood pressure have been contaminated by an impurity that can increase the risk of cancer. As Covid cases drop in Georgia and Florida some states with colder weather see an increase Atlanta Georgia.
Lupin Pharmaceuticals recalled all batches of Irbesartan tablets in 75 mg 150 mg and 300 mg strengths and Irbesartan and Hydrochlorothiazide tablets in 150 mg125 mg. The expansion is the fifth by Torrent involving widely used losartan. Food and Drug Administration FDA.
Food and Drug Administration. Blood pressure pills recalled over cancer risk. I am a writer journalist professor systems modeler computational and.
Details About the Recall. Lupin Pharmaceuticals Inc. Opinions expressed by Forbes Contributors are their own.
Is voluntarily recalling a blood pressure medication that possibly contains high levels of a cancer-causing impurity according to the US. The Medicines and Healthcare products Regulatory Agency MHRA issued a notice. Announced it is voluntarily recalling eight lots of blood pressure medication due to trace amounts of a cancer-causing chemical.
FRAMINGHAM MA-- Batches of Irbesartan Tablets and Irbesartan and Hydrochlorothiazide Tablets made by Lupin Pharmaceuticals Inc. After the companys testing process it detected high levels of a cancer-causing substance in the affected products. A blood pressure medication is being recalled for possibly containing high levels of a cancer-causing impurity according to a recall notice from the US.
A blood pressure medication is being recalled for possibly containing high levels of a cancer-causing impurity according to a recall notice from the US. The UK medicine regulator today issued a recall for 31 batches of. Another blood pressure medication has been recalled over concerns it could contain trace amounts of carcinogens.

High Blood Pressure Medication Recalled Due To Cancer Causing Substance
:strip_exif(true):strip_icc(true):no_upscale(true):quality(65)/d1vhqlrjc8h82r.cloudfront.net/04-19-2019/t_0143e33498c148e98f334211f82e2e15_name_image.jpg)
Blood Pressure Meds Recall Expanded Due To Potential Cancer Causing Ingredient

More Blood Pressure Medication Recalled After Cancer Causing Chemical Found
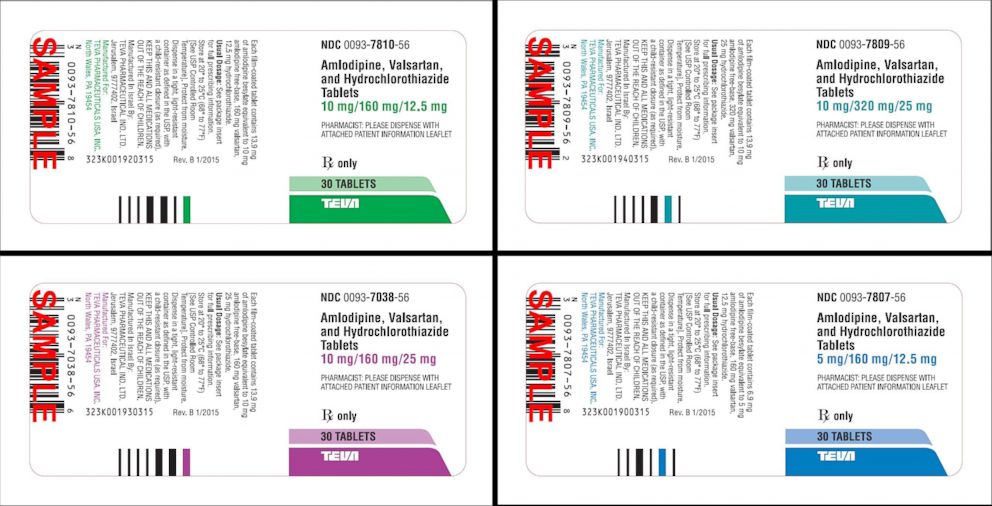
Blood Pressure Medication Recall What You Need To Know Abc News
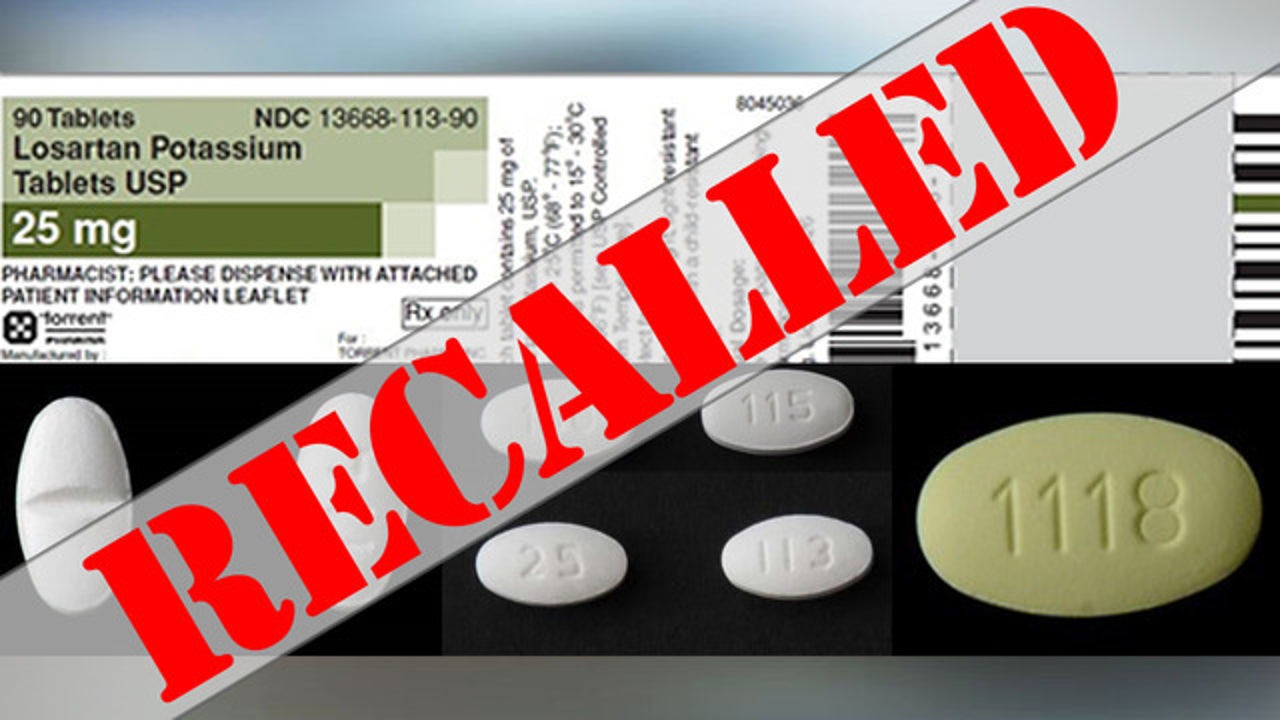
Blood Pressure Medication Recall Expanded After Possible Cancer Causing Impurity Detected Says Fda

More Blood Pressure Meds Recalled Over Cancer Causing Ingredient 10tv Com

Cancer Causing Chemicals Spark Recall Of Blood Pressure Drugs Over Contamination Fears

Blood Pressure Medication Recalled After Trace Amounts Of Cancer Causing Chemical Found Cbs News

Blood Pressure Medication Recalled Over Possibly Containing Cancer Causing Impurity Fox News
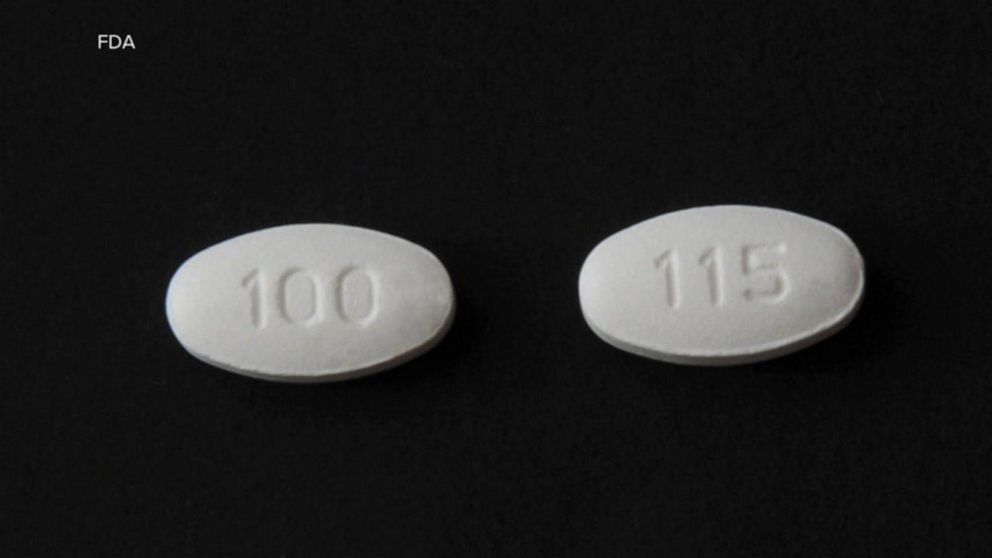
Blood Pressure Medication Recall What You Need To Know Abc News
Another Blood Pressure Medication Recall Due To Cancer Causing Impurity

New Recall Issued For Blood Pressure Meds That Contain Cancer Causing Chemicals Clarksvillenow Com
Valsartan Losartan And Other Blood Pressure Medication Recalls 2018 19

Blood Pressure Medication Recalled For Containing Cancer Causing Substance Abc11 Raleigh Durham
/cloudfront-us-east-1.images.arcpublishing.com/gray/A7A5CIT5GREO7OVZPSG4ZHG2U4.png)

/cloudfront-us-east-1.images.arcpublishing.com/gray/IFRWTBMGXFAAVL36BTAQD77TQ4.png)



