Moderna Booster Shot Timing Immunocompromised - Acr Recommends Booster Dose Of Covid 19 Mrna Vaccine For Patients On Immunosuppressants
The Pfizer vaccine is approved for people 12 or older. Those immunocompromised who have already received two doses of either Moderna or Pfizer-BioNTech should wait at least 28 days after their second dose before receiving their third dose.
Fda Immunocompromised Should Get Covid 19 Vaccine Booster Shots
The Moderna vaccine is approved for people 18 or older.
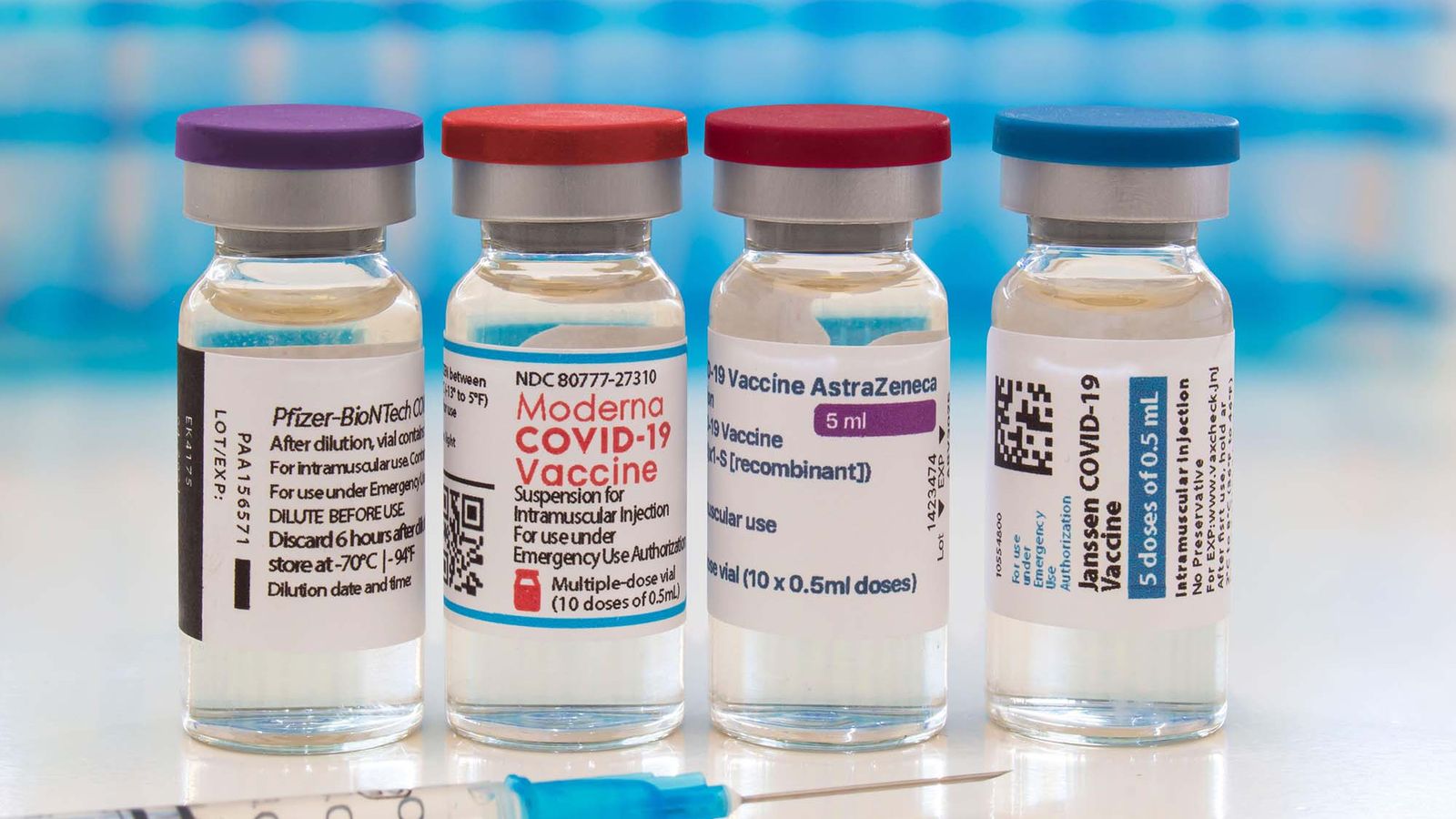
Moderna booster shot timing immunocompromised. Modernas COVID-19 booster vaccine. Immunocompromised people 18 and older who got two shots of Moderna should get a third shot of Moderna. And the CDC recommends getting a booster dose of the vaccine.
FDA authorizes additional Covid-19 vaccine doses for certain immunocompromised people. Third doses are for severely immunocompromised individuals whereas a booster is recommended for individuals 16 or older for Pfizer and 18 or older for Moderna. The Food and Drug Administration is set to amend clearances as soon as Thursday for vaccines from both Moderna and Pfizer Inc.
Both vaccines are administered as a series of two shots. In August 2021 the US. CDC recommends moderately to severely immunocompromised people consider receiving an additional third dose of an mRNA COVID-19 vaccine Pfizer-BioNTech or Moderna at least 28 days after the completion of the initial 2-dose mRNA COVID-19 vaccine series.
People with compromised immune systems who already got two doses of the Pfizer or Moderna vaccines can now get a third shot to boost their protection from COVID-19. Additionally the FDA has not recommended booster vaccines for the general public. Food and Drug Administration FDA adjusted the emergency use authorizations EUAs for the Pfizer and Moderna vaccines to allow for an additional dose to be given to certain.
If you got the Moderna vaccine against COVID-19 you could be in line for a booster shot. The meeting came after the Food and Drug Administrations Thursday evening announcement expanding the emergency use authorization of the two. As for why the booster shots would be recommended at eight months Singer said its not entirely clear but its probably a combination of waning immunity over time and the fact the.
The Food and Drug Administration on Thursday will authorize third doses of Pfizer and Modernas. These individuals can get their booster shot eight months after receiving their second dose of either the Pfizer or Moderna vaccines. However the timing is different.
Food and Drug Administration amended the emergency use authorizations EUAs for both the Pfizer-BioNTech COVID-19 Vaccine and the Moderna COVID-19 Vaccine to allow for the use of an additional dose in certain immunocompromised individuals specifically solid organ transplant recipients or those who are diagnosed with conditions that are considered to have an. Third dose of COVID-19 vaccine recommended for certain immunocompromised individuals OLYMPIA Health care providers can now offer third doses of Pfizer-BioNTech and Moderna COVID-19 vaccines to certain immunocompromised individuals following recommendations from the US. FDA set to authorize extra Covid vaccine doses for immunocompromised patients.
The White House is preparing to give more Americans booster shots as early as Sept. Memorial Hermann said more details will be shared on this. Food and Drug Administration FDA Advisory Committee on Immunizations Practices ACIP.
CNNVaccine makers are preparing for a next possible phase of the Covid-19 vaccine rollout. A third dose may be administered after 28 days for the second dose while a booster is recommended after 8 months from the second dose. Moderna CEO Stéphane Bancel predicted that you might need a booster of the vaccine as soon as eight or nine months after your first round.
The Centers for Disease Control and Prevention says if the matching vaccine. The Pfizer-BioNTech COVID-19 Vaccine is administered three weeks apart and the Moderna COVID-19 Vaccine is administered one month apart. COVID booster vaccine timing in flux as scientists say shot not needed for most.
Today the US. What to know today. Many people have questions about recommendations for additional doses of the COVID-19 vaccine in immunocompromised individuals and the booster shot for the general population.
Approval status who would be eligible and more. An advisory panel to the Centers for Disease Control and Prevention voted unanimously during a Friday meeting to allow some immunocompromised people to receive a third dose of the Pfizer or Moderna vaccines.
Covid 19 Vaccine Boosters May Be Necessary At Some Point Here S What You Need To Know Cnn
Cdc Recommends 3rd Vaccine Dose For Immunocompromised People Shots Health News Npr

Who Among The Immunocompromised Can Get Extra Covid Vaccine Doses

Covid 19 Vaccine Boosters Likely Needed For Immunosuppressed Population

Covid Boosters Cdc Group Weighs Third Shot For Immunocompromised People

Acr Recommends Booster Dose Of Covid 19 Mrna Vaccine For Patients On Immunosuppressants
Covid Booster Shots Everything You Need To Know The Brink Boston University

Covid Booster Shots Everything You Need To Know The Brink Boston University

Moderna Asks Fda To Authorize A Booster Shot Of Its Covid 19 Vaccine Coronavirus Updates Npr
Summit County Covid 19 Vaccine Information Summit County Health Department

Us Cdc Recommends Third Dose Of Pfizer And Moderna Covid 19 Vaccines For Immunocompromised People Aidsmap

New York State Authorizes 3rd Covid Vaccine Dose For Immunocompromised People
Do I Need A Covid 19 Booster Shot 6 Questions Answered On How To Stay Protected

Cdc Approves Booster Covid Vaccine For Immunocompromised Krcg

Hy Vee Offering Third Doses Of Pfizer Moderna Vaccines For Immunocompromised People Who13 Com

Reports Fda Expected To Authorize Covid 19 Vaccine Booster Shots For Immunocompromised Within Days

Booster Shots Alone Won T Protect Immunocompromised People Stat

Covid 19 Booster Shots Now Available For Immunocompromised Here S Who S Eligible Hartford Healthcare Ct
U S Authorizes Third Shot Of Covid 19 Vaccines For The Immunocompromised Reuters